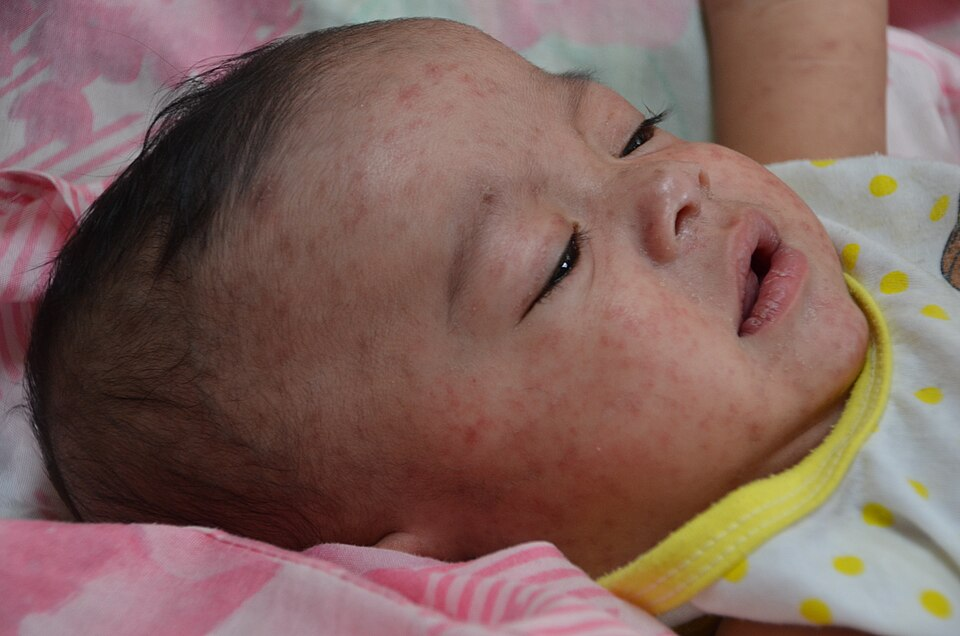
We want to keep you informed about a serious and evolving public health issue: the resurgence of measles in the United States. Measles is not just a rash and fever—it’s a highly contagious virus that can lead to life-threatening complications, especially in children.
🚨 What’s Happening Now?
• As of March 21, 378 measles cases have been confirmed across 15 states—already surpassing the 285 total cases reported in all of 2024.
• So far this year, one child and possibly one adult in the U.S. have died from measles-related complications:
- A school-aged child in Texas
- An unvaccinated adult in New Mexico (under investigation, tested positive after death)
**17% of cases have involved hospitalization
These heartbreaking losses are a stark reminder of what’s at stake.
🧭 How Measles Typically Progresses
Understanding the timeline of illness can help with early recognition:
• Exposure to onset of illness (incubation): Measles symptoms usually appear 6 to 21 days after exposure (on average, 13 days).
• Early symptoms (prodrome): Once symptoms begin, your child may experience fever, fatigue, cough, runny nose, and red eyes for 2–4 days. These are often mistaken for a cold or flu.
• Koplik spots (tiny white spots on the inside of the cheeks) may appear just before the rash.
• Rash appears: Around day 3–5 of illness, a red, blotchy rash starts on the face and spreads downward across the body.
• Fever may spike as the rash peaks. Children can be contagious from 4 days before the rash appears to 4 days afterward.
👶 Why Measles Is So Dangerous for Kids
For every 1,000 children who get measles:
• 1 to 3 children will die
• 1 child will develop encephalitis (brain swelling), which can cause permanent brain damage or deafness
• 200 will develop pneumonia, the leading cause of measles-related death in young children
Complications are even more common in children under 5, those with weakened immune systems, and people who are malnourished or unvaccinated.
💉 Vaccine Effectiveness and Breakthrough Cases
The MMR vaccine (measles, mumps, and rubella) is extremely effective:
• 1 dose = ~95% protection
• 2 doses = ~97% protection
95% sounds very good, and is very good, however….
95% effectiveness still means that 1 in 20 exposed vaccinated individuals will still contract measles! (It’s important to understand statistics)
That said, vaccinated individuals who do get measles usually have mild illness and are much less likely to have severe complications or spread the virus to others.
In this year’s outbreak, a small number of vaccinated individuals have gotten measles, but nearly all severe cases and deaths have been among the unvaccinated.
🎥 A Family’s Story & What to Remember
You can view the interview with the parents of the Texas child who died from measles at the following link:
If you decide to watch this video, please keep in mind:
• Most children will survive measles. About 999 out of 1,000 kids will recover.
• However, 1 in 1,000 children will die—and these are deaths that could have been prevented.
• Many families would prefer to avoid the stress and fear of watching their child struggle with pneumonia, encephalitis, or hospitalization.
In general, if your child becomes ill with breathing problems, your pediatrician (Dr. Homan or Ms. Carmen) may try various supportive treatments—like albuterol or steroids—depending on their symptoms and clinical findings.
Two Treatments have shown (some) effectiveness
Vitamin A: In resource poor countries with malnutrition, vitamin A is definitely helpful. Studies in resource rich countries, like the United States, do show that it might be helpful in children under two years of age with severe measles.
Ribavirin: Has been shown to be helpful in children with severe pneumonia.
🏥 What We’re Doing at Canopy Pediatrics
If we suspect measles in our clinic, we follow strict infection-control protocols to protect your family. If your child develops:
• Fever or Cough, red eyes, or runny nose
AND
• A rash that starts on the face and spreads
Please call us first before coming in. We will guide you on safe next steps.
✅ What You Can Do Right Now
• Make sure your child is fully vaccinated—1 dose of MMR for kids under 4 years of age, and 2 doses of MMR for kids 4 years-and-older.
• If you’re unsure of your child’s vaccine status, we’re happy to help check and catch them up
• Stay informed, especially if traveling or near areas reporting outbreaks
Vaccination is not only about individual protection—it’s how we protect newborns, cancer patients, and others who can’t yet be vaccinated.
MMR Adverse Effects
MMR is extremely safe, but there are known risks. 1 in 40,000 children will develop ITP (immune thrombocytopenenic purprua). This is a temporary dysfunction of the platelets and clotting in the body, thankfully mortality is essentially zero.
Possible Febrile Seizure from the MMR Vaccine
And about 1 in 1,500 kids who receive an MMR vaccine will experience a febrile seizure within 2 weeks of the immunization. It is less frequent with MMR than the MMR-chicken pox combination vaccines (we do not carry the MMR-chicken pox combined vaccine at Canopy Pediatrics).
Febrile Seizures are very scary, but they are generally isolated incidents without future effects
1. Febrile seizures look scary but are generally harmless – They do not cause brain damage, epilepsy, or long-term health issues.
2. They are relatively common – About 2-5% (2-5 out of every 100) of young children will experience a febrile seizure at some point, often triggered by common viral infections.
3. The risk of getting a febrile seizure from a “normal” illness is higher than from vaccines – A child is more likely to have a febrile seizure from a fever caused by a virus like the flu or roseola than from the MMR vaccine.
4. They don’t increase a child’s risk of future problems – Most kids who have febrile seizures go on to develop normally with no increased risk of epilepsy.
5. The MMR vaccine protects against much more dangerous illnesses – Measles itself can cause seizures, encephalitis, and even death. The vaccine prevents these much more severe risks.
(but still, no parent wants to experience a febrile seizure)
I hope this summary has been helpful! Please message me with any questions or comments!
